Cookbook #208: Kitchen Science, Revised Edition, Howard Hillman, Houghton Mifflin Company, Boston, 1989.

The following is a quote from Howard Hillman, found in the foreword to this revised edition of Kitchen Science:
“By popular demand, we’ve expanded Kitchen Science, by 25 percent. It was fun because we love to eat, cook, and experiment (a friend affectionately nicknamed us ‘mad scientists in the kitchen’).”
I love this book! Mad scientists in the kitchen, much like me.
From the preface:
“Find yourself a recipe, and you can cook one dish. Teach yourself the science of cooking, and you can cook creatively forever. Creative cooking requires love, imagination, art, and science.”
I bought this book for myself when I was a working chemist. I even loaned it to some of my students over the years. I am very happy to revisit this book for this blog – Kitchen Science has laid on the shelf way too long. Out of curiousity, I checked to see if Howard Hillman has updated Kitchen Science, and sure enough, there is a revision released in 2003.
The first chapter of Kitchen Science covers cooking equipment, for example: how to choose a good knife and how to sharpen it; the advantages/disadvantages of different types of pans.
The next chapter, cooking methods, covers the usual stove top and oven methods, but with a much more scientific twist. Example: “Heat cooking is chemistry. When you increase the heat, you increase the velocity of the molecules in a food. The greater the speed, the more the molecules collide.These microscopic crashes can alter the molecular structures, creating new molecules and changing the color, flavor, and texture of the cooking food.” He also answers questions like “Is there a difference in temperature between lightly and vigorously boiling water?” (no) and “Does salt raise the boiling point of water?” and “Is braising best done in the oven or on to of the stove?” and “What causes a lid to stick to a pan?”
I learn in the “Meats” chapter that a “bloody red steak” isn’t really bloody, instead, the red color comes from myoglobin, a constituent of muscle. Furthermore, myoglobin undergoes chemical changes as its temperature rises, going from pink to drab brown. Thus, the red color in “blood rare” steaks is actually pink myoglobin. “London broil” has been a popular dish since I was a little kid. Today’s markets sell thick round steak as “London broil”. However, true London broil used to be flank steak, a cut of meat that has long fibers and is taken from the side or “flank” of the cow. After World War II, Americans took up the “fashionable backyard sport of barbecuing”, and since each steer has only two flank steaks, butchers began merchandising the relatively abundant round steak as “London broil”. Hillman discusses the pros and cons of salting meat before or after cooking. And beyond beef, he writes that free range chickens have more flavor because exercise developes more flavor in their muscles.
On the topic of fish, Hillman explains that freshwater fishes have more “small, annoying bones” than marine fishes, because salt water has a higher specific density, thus fish in the ocean have a greater buoyancy and that buoyancy allows them to have a heavier bone structure. Lobster claws are secured with rubber bands because lobsters are cannibals, and unless their claws are disabled they will eat each other while they are held in lobster tanks.
I have often run across recipes that say to “scald milk”. I tend to skip this step, always feeling a bit guilty. But no more! From Kitchen Science: “Scalding has two primary purposes: to kill pathogenic microorganisms and to destroy certain enzymes that would keep emulsifying agents in the milk from doing their thickening job.” But today, this book explains, when you buy pasteurized milk, about the only kind of milk offered in US stores, both of these tasks have already been accomplished. Yay! I can now ignore directions to scald milk!
How about drinking chocolate milk as a source of calcium? Well, chocolate has oxalic acid in it, and this chemical inhibits the digestive system’s ability to absorb calcium. So not such a good idea. Unsalted butter is better for cooking because the salt content of salted butter can vary from brand to brand. Recipes from “virtually all serious cookbooks are based on the use of sweet (unsalted) butter”. I have gradually changed my recipes over the past several years to use sweet butter, and now I know why. Pages 110-112 give a great method for clarifying butter, and I’ll definitely use this method next time a recipe calls for it.
Eggs are the next chapter. To this day, I use the method described in Kitchen Science to boil eggs. First, I use a push-pin to make a hole in the less-pointed end of the egg. You see, after an egg is laid, the yolk and albumen shrink and an air pocket forms between the less pointed end and the shell. When you put the egg in hot water to boil, this area can expand and crack the shell. That’s not only annoying, it makes the boiling water all white and yucky! So I poke a hole in the flatter end of each egg shell, and gently lower the egg into boiling water, and happily watch a stream of air bubble out of the egg. Almost never do these poked egg shells crack in the boiling water. (More on this technique below.)
My interest in chicken eggs may come from my time in graduate school. Here is the title of my master’s thesis: “The effect of methylene bridged monoribonucleotides on transformation of chick embryo fibroblasts by Rous sarcoma virus” (by Patricia Louise Feist, University of Colorado, 1976). For this research project, I had to incubate fertilized chicken eggs for about 9 days in a special small egg incubator (which was re-discovered in the attic storage of the old chemistry building in the 2000s). Each egg had to be placed in the incubator with the flatter end up, otherwise the air sac would break and the whole egg would become infected with bacteria. When you buy a carton of eggs, they should always be sold with the flatter end up. But, I often find them upside down in today’s markets. Perhaps this is because they are sterilized before being sold, a process which oddly enough makes them require refrigeration. In Togo, eggs were not sterilized, and stored at room temperature, and were just fine because unsterilized eggs have a natural protection.
I digress! Back to Kitchen Science. “What is the difference between a fruit and a vegetable?” Here is the excerpt:

Now I think I’ll call tomatoes “fruit-vegetables”.
“Sauces and Thickeners” is the next chapter. I took a cooking class on sauces and the information in this chapter correlates with what I learned there. Emulsified sauces intrigue me. I guess it’s from all those times I shook up solutions in a separatory funnel, and often had a nasty emulsion at the interface of the aqueous and hydrocarbon layers. Emulsions in the lab were a pain in the neck, but an emulsion in a salad dressing is a wonderful thing. Kitchen Science has several “experiments” on making emulsified and other sauces, like Hollandaise, Vinaigrette, and Maltese Falcon Custard, and Mornay Sauce. How fun! I’d like to make them all.
In the “Seasonings” chapter, I learn that kosher salt is better than regular salt for sprinkling on food like corn on the cob because the salt crystals are larger and because they have a more jagged configuration, and thus cling better to food surfaces. “Kosher salt is so named because it was specially developed as an aid for Jews who adhere to kosher dietary laws, one of which requires that as much blood as possible be removed from meat before it is cooked; the characteristics of kosher salt make it better suited for drawing out the blood”. MSG, monosodium glutamate, highlights the flavor of salt, but leaves a peculiar off-flavor. Hot chili peppers are more popular in tropical (hot) zones because they cool the body, perk up the appetite, and add zest to a bland diet (veggies and seafood in the tropics are bland, according to Kitchen Science). Hot chilis are a preservative, swamp the telltale tase of spoilage, reduce the incidence of diarrhea, and aid digestion by accelerating the flow of gastric juices.
“Oils and fats” explains that an oil is a fat, but it is common practice to use the term “fat” for those fats that are in a solid state at room temperature. Shortening can be made from animal or vegetable fat; those based on vegetable oil are made solid by hydrogenation.
I learned something about unbleached flour in the baking chapter. (I also discussed unbleached flour here.) According to Howard Hillman, unbleached flour has a more natural taste because it has not been treated with bleaching chemicals. However, unbleached flour must be aged for several months to strengthen its gluten content; bleaching artificailly ages the flour so it can be sold right away. Thus, bleaching the flour is not only a cosmetic preference demanded by the consumer, as I always thought, but also a way to get a product to market faster. I learn that sifting flour is no longer necessary (yay!), because “Most modern baking recipes are based on the measured volume of unsifted flour, partly because the flour sold today is not as compacted as it was in our grandparent’s day. But even if the flour of yesteryear didn’t need sifting for lightness, it probably would have been best to sift it anyway to remove insects and other impurities.”
Pages 258-262 give and describe a list of about twenty common food additives. Granted, it’s not as complete as J. Michael Lapchick’s book The Label Reader’s Pocket Dictionary of Food Additives, but this list is still useful.
The last chapter is a potpourri of science facts about foods. In this chapter is a 6-page principle-illustrating recipe on how to make a souffle. I think I’ll review this section next time I make a souffle for dinner (one of our favorite meals).
For this blog, I decide to boil eggs. Ha, what can be easier? Who needs a recipe? Well, there are a lot of controversies on how to properly boil an egg. Some start in cold water, for instance. As I stated above, I always use the method described in Kitchen Science. (Please read the first sections of this blog entry to find out why I poke holes in the eggs before boiling.)
Hard Boiled Eggs
Step 1. Bring a pot of water to a rolling boil, then turn the heat down a bit so that it’s boiling but a little calmer. If it is a huge rolling boil, the eggs might hit each other while cooking, increasing the chance that they will break. But it’s important to have the water boiling because this recipe is carefully timed, and you need the water at a predictable temperature.
Step 2. While still in the carton, study the eggs you will cook. The top side should be the flatter end of the egg, the end where the air sac resides.
 I took the eggs out of the carton in the same order and laid them on a cloth with the upper ends to the right for each egg. Note how hard it can be to find the flatter end – sometimes both ends look the same.
I took the eggs out of the carton in the same order and laid them on a cloth with the upper ends to the right for each egg. Note how hard it can be to find the flatter end – sometimes both ends look the same.
 Below is a close-up of these same eggs. I am tilting one egg, flat side up:
Below is a close-up of these same eggs. I am tilting one egg, flat side up:

Poke the flat side of each egg with a pushpin. Two illustrations follow. The first is an illustration I copied from Kitchen Science, where the hashed lined represent the air sac between the egg white/yolk and the egg shell:
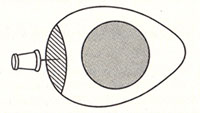
This next illustration is me poking the egg with a pushpin:
 Poked hole:
Poked hole:

Step 3. Gently lower each egg in the boiling water and watch for bubbles coming out of the egg. If air bubbles rather than egg white comes out, you have chosen the correct end. If egg white comes out, quickly remove the egg and save it for a different use.
 As careful as I was to remove the eggs from the carton properly and poke what looked like the flatter end, one of the eggs had been put in the carton upside down was thus in the wrong orientation (the upper left egg in the pattern of six). Instead of bubbles, white goo came out when I lowered it into the boiIing water. I removed it immediately. Here is the gooey egg white coming out of the hole in the upper left egg:
As careful as I was to remove the eggs from the carton properly and poke what looked like the flatter end, one of the eggs had been put in the carton upside down was thus in the wrong orientation (the upper left egg in the pattern of six). Instead of bubbles, white goo came out when I lowered it into the boiIing water. I removed it immediately. Here is the gooey egg white coming out of the hole in the upper left egg:

Step 4: As soon as all the eggs are in the water, set your timer! Kitchen Science says to boil the eggs 12 to 15 minutes if you are at sea level; boiling them longer can make them turn dark green around the edges of the egg yolk.
I have always boiled eggs 20 minutes, but my kitchen is at 5300 feet. Is 20 minutes really the proper time I should use? I will do a little experiment today, and cook 3 of the eggs for 15 minutes, and 3 of the eggs for 20 minutes.
I measured the temperature of the boiling water, and it was 202˚ F.
Step 5: When your timer dings, remove the pot from the stove and immediately place the eggs in cold water to stop the cooking.
Kitchen Science says it is easier to peel the eggs when hot. So, after 5 minutes in cool water, I removed them and peeled them. And yes, the shells came off easily.
I cut each of the 6 eggs in half. In the photo below, the 15-minute eggs are on the top of the plate, and the 20-minute eggs are on the bottom.
 Conclusion: In my opinion, the 15-minute eggs were a bit undercooked. Here is a close-up, with a 15-minute egg on the left, and a 20-minute egg on the right:
Conclusion: In my opinion, the 15-minute eggs were a bit undercooked. Here is a close-up, with a 15-minute egg on the left, and a 20-minute egg on the right:
 Neither has a dark green discoloration on the sides of the yolk.
Neither has a dark green discoloration on the sides of the yolk.
So, the 20 minutes I have always used is the best time to hard boil eggs at my altitude. Maybe I did this experiment years ago, when I first got this book. But it totally makes sense that it will take longer to hard boil eggs if my boiling water is 202˚ F instead of the sea level standard 212˚ F.
It was getting to be lunch time, and I couldn’t resist putting a little salt on an egg and scarfing it up. So good! I made deviled eggs from the rest (added a little mustard and mayonnaise to the yolks, topped with a bit of paprika). We both love deviled eggs. They were gone early the next day.
Simple hard boiled eggs. A good lesson in science, and a good thing to eat.
 I just now realized: This paperback book has the same title and cover photo and publication date as my hardcover book Crockery Cookery. This paperback is from my own collection, while the hard back version was my mother’s. I didn’t mark or note any of the recipes in the paperback. The information on the use of different brands of crockpots is the same in both, but some of the recipes are different. And, the hardcover edition is better illustrated.
I just now realized: This paperback book has the same title and cover photo and publication date as my hardcover book Crockery Cookery. This paperback is from my own collection, while the hard back version was my mother’s. I didn’t mark or note any of the recipes in the paperback. The information on the use of different brands of crockpots is the same in both, but some of the recipes are different. And, the hardcover edition is better illustrated. The recipe calls for beef stew meat, but I have a quantity of pork loin in the freezer so I decide to use that instead of beef.
The recipe calls for beef stew meat, but I have a quantity of pork loin in the freezer so I decide to use that instead of beef. This was very good. I’d make it again!
This was very good. I’d make it again!
 I bought the Handbook of the Nutritional Contents of Foods for myself new or used, I think sometime in the eighties. Around that time, I was developing a basic “eating plan” for myself, or maybe I should say “dieting plan”. You see, for years, I would diet strictly for months, eat sensibly for a short time, overindulge for weeks, then repeat the cycle. I drew up my first plan based on a diet given to me in the early seventies, before I left California. Later,
I bought the Handbook of the Nutritional Contents of Foods for myself new or used, I think sometime in the eighties. Around that time, I was developing a basic “eating plan” for myself, or maybe I should say “dieting plan”. You see, for years, I would diet strictly for months, eat sensibly for a short time, overindulge for weeks, then repeat the cycle. I drew up my first plan based on a diet given to me in the early seventies, before I left California. Later,  One thing I recall about looking up calories in this book is that it was difficult to find the calories in meats. I think maybe it was because I didn’t have a kitchen scale at the time, and would have to estimate the calories from the meat package label, using the amount sold (raw) and estimating the size of the portion I cut for myself.
One thing I recall about looking up calories in this book is that it was difficult to find the calories in meats. I think maybe it was because I didn’t have a kitchen scale at the time, and would have to estimate the calories from the meat package label, using the amount sold (raw) and estimating the size of the portion I cut for myself.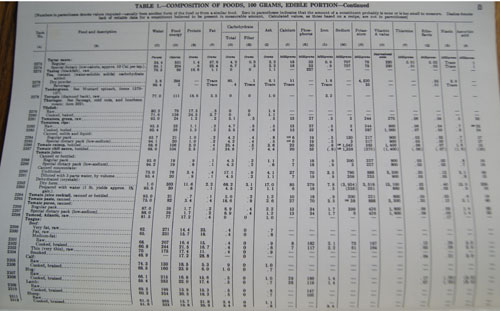
 And it baked into a beautiful loaf. Here is a slice, showing the blue hue and the great texture:
And it baked into a beautiful loaf. Here is a slice, showing the blue hue and the great texture: I sniffed the loaf like I always do. Pee-yoo, it stinks! Something about the smell really turns me away. I ate a big bite and it tasted like it smelled. Yuck.
I sniffed the loaf like I always do. Pee-yoo, it stinks! Something about the smell really turns me away. I ate a big bite and it tasted like it smelled. Yuck. My Simac Pastamaker MX700 was a gift from my husband in 1998. I had been making my own pasta for years, using a Kitchenaid mixer to mix the dough, and a manual Marcato pasta maker to roll the dough and form the noodles (see my post, the
My Simac Pastamaker MX700 was a gift from my husband in 1998. I had been making my own pasta for years, using a Kitchenaid mixer to mix the dough, and a manual Marcato pasta maker to roll the dough and form the noodles (see my post, the  It’s a lot of fun! And time-consuming, yes. A decade or so ago I stored the Pastamaker down in the basement to make room for other things on my countertop. I don’t think I’ve used it since then. Now I have a great excuse to pull it back up, clean it, and make some great pasta. (I love retirement!)
It’s a lot of fun! And time-consuming, yes. A decade or so ago I stored the Pastamaker down in the basement to make room for other things on my countertop. I don’t think I’ve used it since then. Now I have a great excuse to pull it back up, clean it, and make some great pasta. (I love retirement!) My note tells me that I used King Arthur Flour “pasta blend” the last time I made make the dough in the Simac. The reference to the water-egg measuring “cup” means that I used the package instructions for measuring the proper ratio of flour to egg-plus-water. The “cup” was shipped with Simac; it’s a specialized plastic measuring cup with levels marked for water and egg. BUT: these markings do not correlate with ounces, milliliters, or even cups. I never wrote down the exact capacity of that “measuring cup”. Each time I wanted to make pasta in the Simac, I had to locate this single-purpose utensil. I’m glad I wrote that note to myself! – now I know the proper ratio of wet to dry ingredients and can use a regular standard measuring cup.
My note tells me that I used King Arthur Flour “pasta blend” the last time I made make the dough in the Simac. The reference to the water-egg measuring “cup” means that I used the package instructions for measuring the proper ratio of flour to egg-plus-water. The “cup” was shipped with Simac; it’s a specialized plastic measuring cup with levels marked for water and egg. BUT: these markings do not correlate with ounces, milliliters, or even cups. I never wrote down the exact capacity of that “measuring cup”. Each time I wanted to make pasta in the Simac, I had to locate this single-purpose utensil. I’m glad I wrote that note to myself! – now I know the proper ratio of wet to dry ingredients and can use a regular standard measuring cup. I carry the Pastamaker upstairs and find the dies (hidden in a pottery jar upstairs). Some of the dies are crusted with dried dough. Oh, I remember, it’s hard to get the dough out of the tiny slits and holes. Sometimes they clog during use, or the dough doesn’t feed properly through them. Hmmm. I better set aside an afternoon for my re-experiment with my Pastamaker.
I carry the Pastamaker upstairs and find the dies (hidden in a pottery jar upstairs). Some of the dies are crusted with dried dough. Oh, I remember, it’s hard to get the dough out of the tiny slits and holes. Sometimes they clog during use, or the dough doesn’t feed properly through them. Hmmm. I better set aside an afternoon for my re-experiment with my Pastamaker. I decide to make macaroni, because I can make spaghetti and flat noodles any old time in my Marcato.
I decide to make macaroni, because I can make spaghetti and flat noodles any old time in my Marcato. Boil for 30 seconds and check for al dente; boil another 30 seconds if necessary. In Colorado, though, I cooked my macaroni for 3-4 minutes and it was perfect.
Boil for 30 seconds and check for al dente; boil another 30 seconds if necessary. In Colorado, though, I cooked my macaroni for 3-4 minutes and it was perfect.
 I discussed electric fry pans in
I discussed electric fry pans in 
 Look at all the Sunbeam appliances! Mixer (see my
Look at all the Sunbeam appliances! Mixer (see my 
 I liked these! But, my dining partner wasn’t impressed. The Southwestern Chicken was enough for him. I think these would have been good with syrup, as suggested in the recipe. I would like them fried in less oil than I used. This is a good recipe for very easy corn fritters; I just need to figure out how to include them in a dinner – or breakfast – plan.
I liked these! But, my dining partner wasn’t impressed. The Southwestern Chicken was enough for him. I think these would have been good with syrup, as suggested in the recipe. I would like them fried in less oil than I used. This is a good recipe for very easy corn fritters; I just need to figure out how to include them in a dinner – or breakfast – plan.
 Here is my Osterizer mini-jar:
Here is my Osterizer mini-jar: It still works with my current Osterizer blender. I should try it sometime!
It still works with my current Osterizer blender. I should try it sometime!
 Did you catch that last paragraph? “In general, you can be suspicious of all new products, those advertised on television or in magazines, and those in flashy boxes.” Can one even find a package of food these days that is not in a flashy package??
Did you catch that last paragraph? “In general, you can be suspicious of all new products, those advertised on television or in magazines, and those in flashy boxes.” Can one even find a package of food these days that is not in a flashy package??

 I like this: “Needless to say this is just an occasional splurge and one not to be eaten by babies.”
I like this: “Needless to say this is just an occasional splurge and one not to be eaten by babies.”



 The tomatoes need to be peeled, seeded, and chopped, and Beard has directions for this on page 16. In a class at the
The tomatoes need to be peeled, seeded, and chopped, and Beard has directions for this on page 16. In a class at the  I am inspired by Beard’s “Pasta with Beans” recipe on page 90. The ingredients are: white beans, bacon, onions, carrots, herbs, canned tomatoes, and elbow macaroni. I have some great home-cooked
I am inspired by Beard’s “Pasta with Beans” recipe on page 90. The ingredients are: white beans, bacon, onions, carrots, herbs, canned tomatoes, and elbow macaroni. I have some great home-cooked 
 Beard is big on cheese. But, I don’t have a lot of good, chunk Parmesan cheese in my refrigerator – and I’ve decided to make this meal with what I have on hand. I sniff all my cheeses, grate up the last (hard) chunk of Parmesan, add a bit of another sharp white cheese, and use the last of my pre-shredded Parmesan.
Beard is big on cheese. But, I don’t have a lot of good, chunk Parmesan cheese in my refrigerator – and I’ve decided to make this meal with what I have on hand. I sniff all my cheeses, grate up the last (hard) chunk of Parmesan, add a bit of another sharp white cheese, and use the last of my pre-shredded Parmesan. I made a loaf of
I made a loaf of 


 And as in the 1955 edition, it is a woman who is doing the cooking, or at least reading the cookbook.
And as in the 1955 edition, it is a woman who is doing the cooking, or at least reading the cookbook. The first section is Appetizers and Spreads. Mother marked several: Crab Meat Canapes, Salami-Julienne Spread, and Cheese Onion Balls. These are all made with cream cheese, cottage cheese, and/or sour cream, with additions of canned or frozen products. During the 50s, and especially during the cocktail hour, these types of appetizers were a mainstay of American cookery.
The first section is Appetizers and Spreads. Mother marked several: Crab Meat Canapes, Salami-Julienne Spread, and Cheese Onion Balls. These are all made with cream cheese, cottage cheese, and/or sour cream, with additions of canned or frozen products. During the 50s, and especially during the cocktail hour, these types of appetizers were a mainstay of American cookery. Here is a photo of some of the appetizers:
Here is a photo of some of the appetizers: Next is Salads. Mother put a check mark on “Carrot and Red Cabbage Salad”. I thought about making this salad, since I like the combination of carrots and cabbage, but this recipe has more sour cream than I think I’d like. A “Full Meal” salad mixes leftover cooked meat with canned or fresh vegetables and a lot of cottage cheese and sour cream. Not for me. Nor are the molded salads. I guess this whole chapter is just not for me!
Next is Salads. Mother put a check mark on “Carrot and Red Cabbage Salad”. I thought about making this salad, since I like the combination of carrots and cabbage, but this recipe has more sour cream than I think I’d like. A “Full Meal” salad mixes leftover cooked meat with canned or fresh vegetables and a lot of cottage cheese and sour cream. Not for me. Nor are the molded salads. I guess this whole chapter is just not for me!


 Next is a rave for all the good things about yogurt, hoop cheese, and milk. “Looking for New Ideas? Ways to use cottage cheese” lists a page of ideas. The following one is my favorite. “The men like this with old-fashioned hot potato salad.”
Next is a rave for all the good things about yogurt, hoop cheese, and milk. “Looking for New Ideas? Ways to use cottage cheese” lists a page of ideas. The following one is my favorite. “The men like this with old-fashioned hot potato salad.” “Looking for New Ideas? Ways to use sour cream” is next, and here is my favorite from that list. “Gourmets babble?”
“Looking for New Ideas? Ways to use sour cream” is next, and here is my favorite from that list. “Gourmets babble?”
 The first issue I need to address in baking these muffins is the “2 cups prepared biscuit mix”. Biscuit mix, or “Bisquick”, was a staple in American kitchens in the 1950s. It is still available today. Should I purchase a box of Bisquick to make this recipe? I think not. I have no other use for it, and in general, I like cooking from scratch, so that I know my ingredients. Luckily, at some time in the recent past I had copied from the web search results for a substitution for biscuit mix:
The first issue I need to address in baking these muffins is the “2 cups prepared biscuit mix”. Biscuit mix, or “Bisquick”, was a staple in American kitchens in the 1950s. It is still available today. Should I purchase a box of Bisquick to make this recipe? I think not. I have no other use for it, and in general, I like cooking from scratch, so that I know my ingredients. Luckily, at some time in the recent past I had copied from the web search results for a substitution for biscuit mix: These were a lot better than I expected! I was concerned that the cherries would not lend enough flavor to the muffins, but I was very wrong. Also, I often use vegetable oil in muffins, but used butter in these. Same calories, but what a difference in texture! These almost taste like pie crust.
These were a lot better than I expected! I was concerned that the cherries would not lend enough flavor to the muffins, but I was very wrong. Also, I often use vegetable oil in muffins, but used butter in these. Same calories, but what a difference in texture! These almost taste like pie crust. This booklet of 26 recipes must have come with our Weber gas grill. I have forgotten all about this cookbook, since soon after getting the grill I purchased Weber’s Real Grilling, a large cookbook with tons of recipes.
This booklet of 26 recipes must have come with our Weber gas grill. I have forgotten all about this cookbook, since soon after getting the grill I purchased Weber’s Real Grilling, a large cookbook with tons of recipes.
 These tasted great! The yogurt marinade makes them not only well seasoned, it also makes them very tender. (I put the blackened side down for the photo. They would have been so much prettier if I hadn’t tried something experimental!)
These tasted great! The yogurt marinade makes them not only well seasoned, it also makes them very tender. (I put the blackened side down for the photo. They would have been so much prettier if I hadn’t tried something experimental!)

 I took the eggs out of the carton in the same order and laid them on a cloth with the upper ends to the right for each egg. Note how hard it can be to find the flatter end – sometimes both ends look the same.
I took the eggs out of the carton in the same order and laid them on a cloth with the upper ends to the right for each egg. Note how hard it can be to find the flatter end – sometimes both ends look the same. Below is a close-up of these same eggs. I am tilting one egg, flat side up:
Below is a close-up of these same eggs. I am tilting one egg, flat side up:

 Poked hole:
Poked hole:
 As careful as I was to remove the eggs from the carton properly and poke what looked like the flatter end, one of the eggs had been put in the carton upside down was thus in the wrong orientation (the upper left egg in the pattern of six). Instead of bubbles, white goo came out when I lowered it into the boiIing water. I removed it immediately. Here is the gooey egg white coming out of the hole in the upper left egg:
As careful as I was to remove the eggs from the carton properly and poke what looked like the flatter end, one of the eggs had been put in the carton upside down was thus in the wrong orientation (the upper left egg in the pattern of six). Instead of bubbles, white goo came out when I lowered it into the boiIing water. I removed it immediately. Here is the gooey egg white coming out of the hole in the upper left egg:
 Conclusion: In my opinion, the 15-minute eggs were a bit undercooked. Here is a close-up, with a 15-minute egg on the left, and a 20-minute egg on the right:
Conclusion: In my opinion, the 15-minute eggs were a bit undercooked. Here is a close-up, with a 15-minute egg on the left, and a 20-minute egg on the right: Neither has a dark green discoloration on the sides of the yolk.
Neither has a dark green discoloration on the sides of the yolk. No author, no publisher, no publication date! This is the only cookbook in my collection with none of that information. It is a
No author, no publisher, no publication date! This is the only cookbook in my collection with none of that information. It is a  I read through this entire book, and am impressed with the author’s work. She (I assume it was a woman, at least) set up each section with an entire menu of recipes for the particular international cuisine. She referenced a sauce from one section (a raspberry sauce) that might go with a recipe from another section (a pecan torte). Because of the consistent writing style and the cross-references, I think this book was all written by one person, although she may have collected ideas from friends of different cultures. The recipes call for canned or processed items, like bean sprouts, canned shrimp and crab, and cake mixes; canned coconut milk was not available; there are several gelatin salad recipes; and microwaves and food processors are not mentioned. All of these facts lead me to believe it was produced in the sixties or seventies. Plus, it was prepared on a typewriter, not a computer.
I read through this entire book, and am impressed with the author’s work. She (I assume it was a woman, at least) set up each section with an entire menu of recipes for the particular international cuisine. She referenced a sauce from one section (a raspberry sauce) that might go with a recipe from another section (a pecan torte). Because of the consistent writing style and the cross-references, I think this book was all written by one person, although she may have collected ideas from friends of different cultures. The recipes call for canned or processed items, like bean sprouts, canned shrimp and crab, and cake mixes; canned coconut milk was not available; there are several gelatin salad recipes; and microwaves and food processors are not mentioned. All of these facts lead me to believe it was produced in the sixties or seventies. Plus, it was prepared on a typewriter, not a computer.
 Hungary: Hungarian Gulyas (like a goulash), Wilted Cucumbers, Cole Slaw, Cream Apples.
Hungary: Hungarian Gulyas (like a goulash), Wilted Cucumbers, Cole Slaw, Cream Apples.

 I put the rolls on 2 parchment-lined half sheet pans and let them rise about 20 minutes (my kitchen was warm). I learned from experience that the rolls bake up best if you tuck the pointy ends under the rolls.
I put the rolls on 2 parchment-lined half sheet pans and let them rise about 20 minutes (my kitchen was warm). I learned from experience that the rolls bake up best if you tuck the pointy ends under the rolls.
 A “yum” for this recipe! These are soft and tasty, and fun to make. The bread machine method worked great. I put some of the rolls in the freezer for later use. Yes, I’d make these again!
A “yum” for this recipe! These are soft and tasty, and fun to make. The bread machine method worked great. I put some of the rolls in the freezer for later use. Yes, I’d make these again!